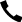Six major trends in the priority domain of cardiac instrumen
Wireless pacemakers receiving attention
During the American Heart Rhythm Society (HRS) conference held in Chicago this year, lead-free pacemakers have long been a hot topic, but it is not surprising to see the estimated market potential of this technology.
From Needham& Company analyst MikeMatson pointed out in a recent report that the global potential market value of lead-free pacemakers is approximately $1.2 billion, with a market penetration rate of 100%. But at the same time, he also stated that the price premium of the device may limit market penetration, especially in the European market. For example, Medtronic’s Micra cordless pacemaker is priced at $10000, and Matson stated that this price is approximately twice the price of a standard pacemaker. Micra was approved by the FDA for listing in April 2016, but due to limited reimbursement, initial sales were slow. But since patients eligible for medical insurance reimbursement in March, this device is now expected to become a driving force for market growth.
At the American Heart Rhythm Society (HRS) conference, Medtronic presented positive data on Micra after its approval for marketing, including 795 patients from 97 centers worldwide. The results showed that although approximately 87% of the participating doctors were novices with no prior experience in Micra implantation, the success rate of implantation of the device within 30 days was 99.6%, and the incidence of major complications was only 1.5%.
Boston Science CorporationScientific has also grown with the development of its Empower lead-free pacemaker (as shown in the slide). The animal research data presented at the HRS conference indicates that the device can provide appropriate pacing treatment and receive communication signals from the company’s subcutaneous implantable defibrillator (S-ICD). These research findings are also published in the Journal of the American College of Cardiology: Clinical Electrophysiology, demonstrating the feasibility of wire free pacemaker design and modular systems.
Among the 40 animals studied, 39 were successfully implanted with the device, of which 23 were tracked up to 90 days after implantation. Its main objective is to study the in vivo communication between S-ICD and lead-free pacemakers, as well as the implementation of anti tachycardia pacing therapy. In 401 attempts, the communication success rate between S-ICD and lead-free pacemaker was 99%, and the success rate of anti tachycardia pacing during communication reception was 100%.
Abbott’s acquisition of St JudeMedical later acquired the Nanostim lead-free pacemaker, but encountered obstacles on the road to FDA approval due to some technical issues with the device.
CRT devices are becoming increasingly intelligent
Medtronic is accelerating the launch of a new type of MR quadrupole cardiac resynchronization therapy pacemaker (CRT-Ps), which has recently received FDA approval. Percepta Quad CRT-P MRISureScan, Serena Quad CRT-P MRISureScan, these new devices are designed to provide clinicians with more personalized treatment plans, and patients implanted with one type of pacemaker can receive MRI scans in 1.5 or 3 Tesla machines. The CRT-P series received CE in FebruaryCertification.
David, MD, Vice President and General Manager of Medtronic Heart Failure BusinessSteinhaus told Qmed that the new platform includes a chipset that allows the company to iterate on the platform faster than before. In addition, these instruments are equipped with all additional features derived from Medtronic CRT-D.
During the HRS conference, Boston Science was relatively close to obtaining FDA approved ResonateICD devices have received attention. Matson emphasized four main features of the new device in his report and stated that these features will help the company win market share in the ICD market:
Resonate has EnduraLife battery technology, providing a longer lifespan, which Matson says is twice the battery life of its competitor ICD.
Before the fourth quarter, Resonate expects to conduct MRI condition labeling before FDA approval.
Matson stated that Resonate will provide SmartCRT treatment, including quadrupole pacing, intelligent delay, and MultiSite pacing, while also providing a lifespan of up to 13.3 years.
Resonate’s HeartLogic feature utilizes Boston Scientific’s heart failure diagnostic algorithm, which has shown a sensitivity of 70% in heart failure event detection, with a median of 34 days in advance.
The data presented at the HRS conference also showed that Boston Scientific’s subcutaneous implantable defibrillator (S-ICD) system has received good feedback in the market. The results of the company’s approved post market study published on HRS showed that 98.7% of evaluated patients effectively terminated arrhythmia after treatment, and the postoperative 30 day complication free rate was 96.2%.
Matson said, "We believe these results demonstrate the potential for widespread application of S-ICD, rather than just being a niche product."
Balloon catheter ablation is becoming increasingly popular
Analyst Matson pointed out that Medtronic’s Arctic FrontThe Advance cryoballoon catheter ablation system continues to gain market share, with an estimated annual sales of over 600 million US dollars. Matson also stated that this growth is mainly attributed to expanding the market to electrophysiologists (EPs) who have not received training in catheter radiofrequency ablation, providing a simpler operating process and eliminating the hassle of having EPs receive training in catheter radiofrequency ablation.
The successor to this priority domain, CardioFocus, obtained FDA approval last year for the Heartlight endoscopic guided laser autofocus ablation system.Heartlight also uses a balloon based system designed to be easier to use than conventional RF catheters.
Although there is no evidence yet that CardioFocus is gaining market share from Medtronic, if Boston Scientific, Johnson&JohnsonThe acquisition of CardioFocus by large companies such as Scientific and Abbott may pose a greater threat to Heartlight. Analysts also pointed out that there are other balloon based ablation catheters under development, including ApamaMedical’s RF energy balloon.
TAVR is becoming increasingly popular
EdwardsLifesciences continues to dominate the market for transcatheter aortic valve replacement (TAVR). The data released by the company during the EuroPCR conference confirms that EdwardsThe clinical efficacy of using the Centera valve for TAVR treatment in patients. These new 30 day results are the first time the company has released data on self expanding valves.
Edwards pointed out that for patients with severe aortic valve stenosis at high risk of cardiac surgery,The survival rate of the Centera valve is 99%, the stroke disability rate is 2.5%, and the pacemaker implantation rate is 4.9%, which is a relatively low incidence rate reported in multicenter clinical trials of self expanding valves.
In addition, the company stated that the incidence of moderate perivalvular leakage (PVL) in patients was 0.6%, and no severe PVL was observed.All 203 patients in the CENTURA-EU study were treated via the femoral artery approach, with the majority of patients (174 cases) receiving treatment under conscious sedation.
Edwards designed a relatively new valve that can be repositioned and retrieved, and this device can be operated through a low frame 14 FrenchThe conveying system is designed with an electric handle for stable valve placement.
Edwards stated that the CenteraValve is expected to receive CE certification in the fourth quarter.
In addition, during the Cardiovascular Intervention Conference (EuroPCR) hosted by the European Society of Cardiology, Edwards reported on sources cited in source 3The new year’s data from the Registry (SAPIEN Aortic Biovalve European Use Results Registry) shows good patient outcomes, high survival rates, and low incidence of stroke and periventricular leukomalacia (PVL).
The post market study included 1946 patients. The one-year survival rate of patients treated through the femoral artery approach is 88.2%, and the stroke disability rate is only 1.1%. Edwards pointed out that the incidence of moderate PVL in these patients was 2.7%, and no severe PVL was observed. The company stated that the majority of registered patients received treatment through the femoral artery approach, and more than half of the patients received treatment under conscious sedation.From July 2014 to October 2015, patients were enrolled in 80 research centers across 10 regions, and the company plans to follow them up for five years.
Boston Scientific has had a tough time finding its place in the TAVR market, but according to key research results presented by EuroPCR, the company’s Lotus valve system is superior to Medtronic’s CoreValve platform in terms of one-year efficacy.
The company stated that the REPRISE III trial is the first head to head criticality study comparing two different TAVR platforms.This multicenter, randomized, controlled trial included 912 patients from the United States, Europe, Canada, and Australia who underwent high-risk or extremely high-risk heart valve replacement surgery for severe aortic valve stenosis.
Although Lotus outperforms CoreValve in PVL for one year, it has a higher pacemaker implantation rate (29.1%) after surgery compared to CoreValve (15.8%) in terms of disability caused by moderate wind.
Technology is reducing the incidence of stroke
W. L. Gore& Associates presented the data from the REDUCE study report at the European Stroke Organization Conference in Prague, which was conducted simultaneously with EuroPCR.According to this study, compared to using antiplatelet therapy alone, Gore’s ventricular septal occluder combined with antiplatelet therapy can reduce the risk of recurrent stroke by more than 76%.
Gore is chasing Abbott, who is acquiring St Jude Medical inherited the Amplatzer device, and the FDA approved the same use of the Amplatzer device last year.Gore stated that he plans to submit the data for this study to the FDA before the end of this year.
Another technology that can effectively reduce stroke is the Watchman left atrial appendage occlusion (LAAC) device from Boston Scientific. The company’s EWOLUTION registration results show that its implantation success rate is high, the incidence of ischemic stroke and major bleeding is low, and it can effectively reduce the incidence of stroke in non valvular atrial fibrillation patients.
Matson said in his report, "In the United States, Watchman remains a conduit delivery type LAAC device, and we expect these data to help sustain its significant growth."
FFR can play a new role in the catheter chamber
During a recent breakthrough clinical trial conference conducted during EuroPCR, researchers at Erasmus Medical Center released early results of a physician initiated FFR Search registration, revealing the potential new role of blood flow reserve fraction (FFR) in the catheterization chamber.
Quickly exchange FFR using Avist Medical Systems NavvusMicrocatheters, researchers have found that FFR based on microcatheters can be used in various clinical environments, including acute coronary syndrome and ST segment elevation myocardial infarction. After PCI, almost half of patients have an FFR below 0.90.These early results did not have a significant impact on the 30 day clinical outcomes, but patients will now be followed up to the primary endpoint of two years to make the actual FFR values after clinical PCI clearer.
More than 1000 stable angina or acute coronary syndrome patients receiving PCI treatment were continuously enrolled at the Erasmus Medical Center in Rotterdam to determine the relationship between FFR values after PCI and clinical outcomes, measured at 30 days, 1 year, 2 years, and 5 years.In an attempt to replicate real-world clinical practices, the research protocol prevented operators from using other optimization techniques after initial stent placement.
Nicolas M. Van, Principal Researcher and Director of Interventional Cardiology at Thoraxcenter, Erasmus Medical CenterDr. Mieghem said, "The preliminary data of FFR Search may significantly expand its application in the laboratory in the future, and we also hope to see important results for the main endpoint indicators over the next two years."
Tom, President and CEO of Acest Medical SystemsMorizio stated that the company’s differentiated microcatheter technology aims to enable doctors to perform rapid FFR measurements before, during, and after intervention therapy, while maintaining the position of their guide wires. MorizioWe believe that compared to other FFR measurement modes (including pressure guided wire based FFR), we are particularly suitable for this new role of FFR
Gore is chasing Abbott, who is acquiring St Jude Medical inherited the Amplatzer device, and the FDA approved the same use of the Amplatzer device last year.Gore stated that he plans to submit the data for this study to the FDA before the end of this year.
Another technology that can effectively reduce stroke is the Watchman left atrial appendage occlusion (LAAC) device from Boston Scientific. The company’s EWOLUTION registration results show that its implantation success rate is high, the incidence of ischemic stroke and major bleeding is low, and it can effectively reduce the incidence of stroke in non valvular atrial fibrillation patients.
Matson said in his report, "In the United States, Watchman remains a conduit delivery type LAAC device, and we expect these data to help sustain its significant growth."