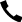留置针固定贴厂家直供按需定制
【产品名称】: 自粘性伤口护贴
【规格型号】:Ⅰ型、Ⅱ型、Ⅲ型、Ⅳ型
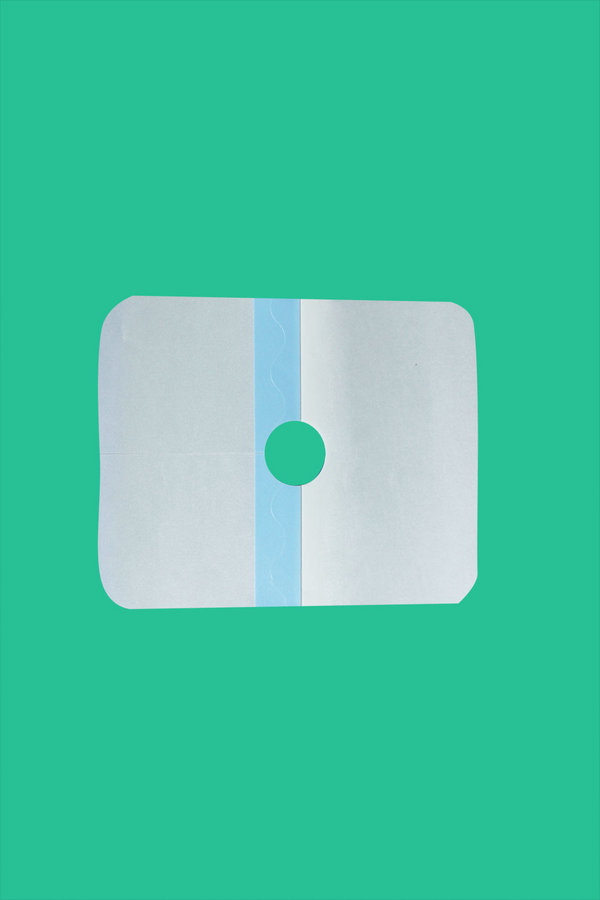
【主要结构组成】:自粘性伤口护贴根据材质不同可分为四个型号:由水刺法非织造布、吸收芯垫、离型纸制成为I型;由水刺法非织造布、离型纸制成为II型;由透明薄膜胶带、吸收芯垫、离型纸制成为III型;由透明薄膜胶带、离型纸制成为IV型。
【产品性能】:1、基布应色泽一致、薄厚均匀、表面洁净平整,无脱胶、污渍、杂质、破损和漏胶等现象。
2、切边整齐,吸收芯垫置于护贴的正中位置,允差±5mm,无自然脱落现象。
3、基布应平整地附着于保护离型纸上,无翻边、无卷边和叠边现象。
4、离型纸应完全覆盖在护贴的基布上,无基布、吸收芯垫外露现象。
5、持粘性:按YY/T 0148-2006 附录B中第B.2章试验时,在烘箱内试验期间,贴于不透钢板 的顶端下滑应不超过2.5mm。
6、剥离强度:按YY/T 0148-2006 附录B中第B.2章试验时,粘贴胶带每1cm宽度所需的平均力应不小于1.0 N。
7、基布的含胶量:粘胶层的含胶量应≥35g/m2。
8、吸收芯垫吸水性:其饱和吸水比应>1:6。
9、护贴应无菌,无菌有效期两年。
10、经环氧乙烷灭菌的护贴,其环氧乙烷残留量应不超过10μg/g。
11、护贴对人体皮肤应无刺激。
12、护贴对人体皮肤应无迟发型超敏反应。
13、护贴细胞毒性应不大于2级。
【适用范围】:用于手术切口及留置动、静脉导管时贴敷皮肤用及术后或外伤创面贴敷, 婴儿肚脐口创口保护。
【使用方法】:打开包装,撕去隔离纸贴于切口的覆盖。
【注意事项】:本品仅限一次性使用,用后销毁。本品经环氧乙有 效期两年。包装破损严禁使用。
【生产日期】:见合格证或包装封口处。
【生产批号】:见合格证或包装封口处。
【失效日期】:见合格证或包装封口处。
【禁忌症】:暂无发现
【贮 存】:在相对湿度不超过80%,无腐蚀性气体、清洁、 通风良好的库房内。

[product name]: self adhesive wound care
[specifications] type I, II, III, IV
[main structure composition]: self adhesive wound patch can be divided into four models according to different materials: spunlaced nonwovens, absorption pads, and off type paper made into I type; II type by spunlace nonwovens and off type paper system; III type by transparent film tape, absorption pad, off type paper, transparent film tape, The form paper is IV.
[product performance]: 1, the base cloth should be uniform in color, uniform in thickness, clean and smooth in appearance, no degumming, stains, impurities, breakage and glue leakage.
2, trimming trimming, absorption core pad placed in the middle of the protective pad, tolerance difference of + 5mm, no natural shedding phenomenon.
3, the base cloth should be attached to the protection of the type paper, without flanging, no curling and overlapping. 4, the release paper should be completely covered on the base of the protective clothing, without the base cloth and the absorbing core mat.

5, sticky: according to chapter YY/T B.2 of appendix B of test 0148-2006, during the oven test, the top of the steel plate should be slipped below 2.5mm.
6, peel strength: according to the chapter B.2 of appendix B of YY/T 0148-2006, the average force required for sticking tape should be less than 1 N per 1cm width.
7. The amount of glue contained in the base cloth: the adhesive content of the adhesive layer should be more than 35g/m2.
8, absorbing core pad water absorption: its saturated water absorption ratio should be > 1:6.
9. The protective paste should be aseptic and the aseptic period is two years.
10. The ethylene oxide residue of ethylene oxide sterilized patch should not exceed 10 g/g.
11, the protective paste should not be irritating to the skin of the human body.
12. There should be no delayed hypersensitivity to human skin.
13. The cytotoxicity of the paster should not be more than 2.
[scope of application]: apply for skin incision with surgical incision and indwelling catheter, and post operation or wound surface application.
[use method]: open the package, tear off the isolation paper and stick it to the coverage of the incision.
[note]: This product is restricted to one time use and destroyed after use. This product is valid for two years by epoxy B. The breakage of the packing is strictly prohibited.
[production date]: see the certificate or seal.
[production batch number]: see the certificate or seal.
Expiration date: see the certificate or seal.
[contraindication]: no discovery for the time being
Storage: in a storeroom without corrosive gas, clean and well ventilated in a relative humidity of not more than 80%.